|
Professor Pietro Cicuta
Professor of Biological Physics
Telephone: 01223 337462 |

|
|
Our work in the last few years has increasingly focused on living systems. Our main efforts at the moment are on: |
|
|
|
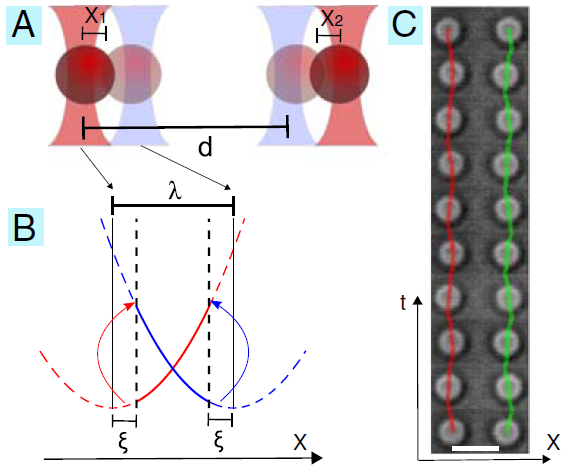
Illustration of the model experimental system we have developed to study the hydrodynamic interaction of colloidal oscillators. |
An experimental model system has been developed using feedback-controlled optical tweezers. Driven oscillations of colloidal particles, of fixed amplitude but free inphase, are achieved. The trap alternates between two minima with a geometric switch triggered by the position of the colloidal particle. In the figure, (A) and (B) illustrate the experimental parameters. The distance between trap pairs is in the range 4 to 40 micron. (C) shows timelapse images of two particles locked in antiphase. Particle positions are overlayed on the image sequence. Antiphase motion can be seen. This result is from our first experiment, published in PNAS in 2010. Since then we have studied more general geometric conditions, including many oscillators, and the effect of changing the driving potential shape from harmonic to other forms.
Synchronisation of colloidal rings
An open problem in biology and physiology is how the motile cilia coordinate their beating to generate fluid flow in the airways and other ciliated tissues, or to enable the motility of microorganisms. The difficulty and hence the challenge for physicists, lies in the fact that the dynamics is non-linear, both at the level of each cilium, and in the coupling between them. Solving this would have impact in medicine, since establishing a link between the behaviour of the single filament and the collective motion would allow us to diagnose readily between various cilia pathologies.
|
|
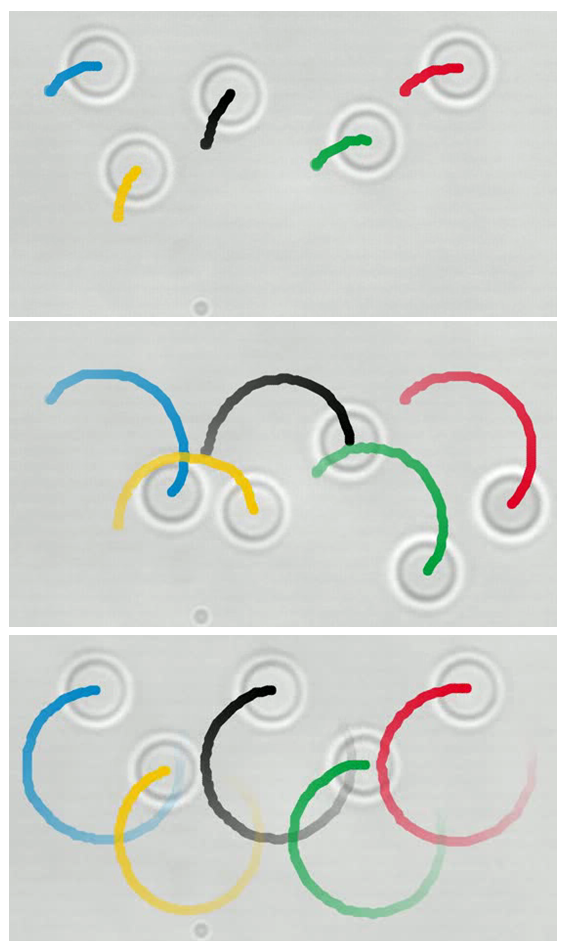
Five colloidal beads are moved with optical traps, with fixed force. They start at random angular positions, and self-coordinate to rotate in-phase. Figure shows three moments of an experiment. |
In the last few years we have been performing model experiments using systems of colloidal scale particles driven by optical traps [J.Kotar et al., Proc. Natl. Acad. Sci. 107, 7669-7673 (2010); N.Bruot et al., Phys.Rev.Lett. 107, 094101 (2011)].
As a development of our work, with Nicolas Bruot and Jurij Kotar we are investigating a new model system proposed in [T.Niedermayer et al., Chaos 18, 037128 (2008)] in which particles are pushed with constant force along pre-defined closed trajectories (orbits). Since only the force is constant, the phase at which the particle travels around the orbits is free: These are phase oscillators. Through the fluid flow, they are coupled, and we see that they exhibit synchronisation.
We have performed a variety of experiments on this system. One particularly captivating arrangement is five beads, as seen in the image evolving in time: The five beads, each a few micrometers in diameter, are initially held by optical tweezers in random positions. Then each one is pushed, on a pre-defined circular trajectory, with constant force. In addition to the tweezers' constant force, they feel the fluid flow from neighbours, and after a couple of orbits they are synchronised, and proceed to rotate thereafter in-phase (last panel).
See the full movie in real time here .
Studying the synchronized states in these simple model systems is helping us to understand the more complex behaviour observed in nature, got us ready for the 2012 Olympic Games.
2. Biological Physics of Bacteria
The most recent research line in our group aims to understand better the growth and the regulation processes within micro-organisms.
Local packing of the chromosome from loci motility
|
|
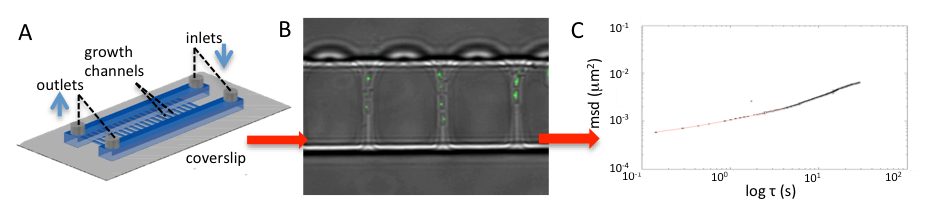
Left panel shows a schematic of the channel device used to grow and image bacteria. Sustained exponential growth is achieved, and can be maintained for a few days. Central panel shows an image in which fluorescent (green) dots are visible inside bacteria; the light is emitted from GFP bound to one particular chromosomal locus. The dots can be traced in time, and the mean square displacement is obtained (right hand panel). Watch this space for the results of this! |
The main question we faced recently, in an HFSP funded collaboration with Marco Cosentino Lagomarsino, Bianca Sclavi, Kevin Dorfman, and with Gillian Fraser in Pathology, concerned the physical structure of the bacterial nucleoid (the genome plus associated bound proteins), and how this in turn affects the fundamental biological processes.
We focus now particularly on gene expression. This research brings together a key biological processes (replication), with polymer physics, and cutting edge microfluidics and imaging technology.
With Madan Babu and Mario Nicodemi we organised in Sept 2011 a very successful meeting on Quantitative Methods in Gene Regulation . A second edition took place in 2013 (with Marco Cosentino Lagomarsino, Mario Nicodemi and Sarah Teichmann), a third and fourth in 2015 and 2017 also with Oliver Stegle and Sebastian Ahnert. The 2019 edition (with Marco, Martin Howard and Alison Smith) will return to London, in the new IOP building.
3. Model and Cell Membranes, Liquid interfaces and monolayers, particle rafts
|
|
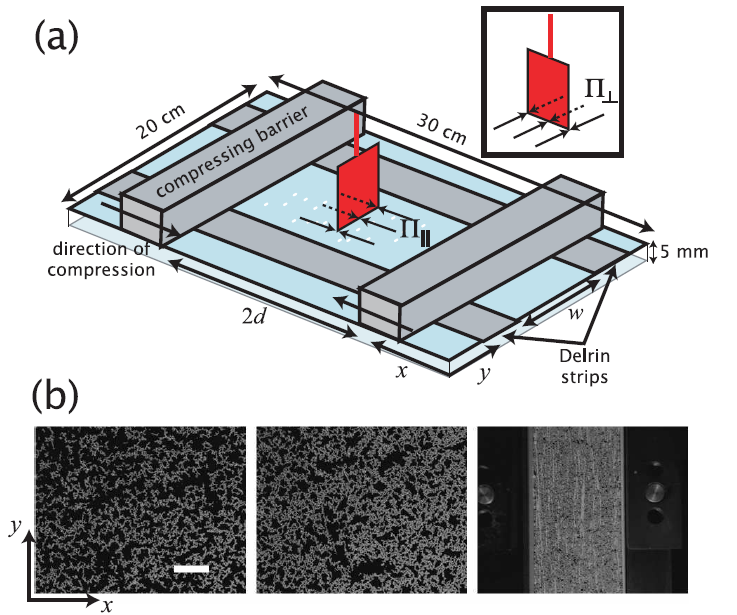
Diagram of customised Langmuir trough setup, used to study stress propagation in two dimensional layers. Images show rafts of large and rough-shaped rubber particles. Recent work has shown that stress is transmitted through these layers in a similar way as in piles of grains. |
Model Membranes
|
|
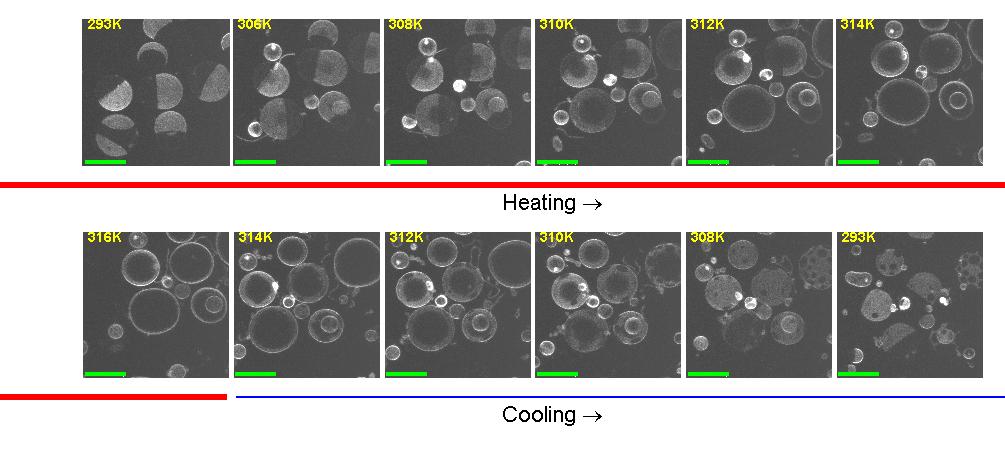
Ternary mixtures of phospholipids and cholesterol phase separate below a critical temperature. Phase separation is imaged in fluorescence, thanks to the preferential partitioning of a fluorescent dye into one phase. This process may have a role in regulating biological activity at membranes. |
Membrane mechanics is characterised by bending, shear and compression moduli. Furthermore, multicomponent membranes can phase separate, and in this case each phase has different moduli, and there is a line tension between the coexisting phases. Working with giant vesicles that mimic the composition of biological cell membranes, we are trying to understand the coupling between the phase behavior and the membrane properties. We are also still active to explore the consequences in cell biology of "critical lipidomics", i.e. the finding (with Sarah Veatch) that the plasma membrane of cells has a lipid composition poised close to liquid-liquid phase separation.
Functionalised and Functional Membranes
In the period 2014-2019 we have developed really interesting systems based on combining the lipid membrane degrees of freedom, with DNA nanotechnology. The DNA binders allow to tune adhesion in specific and information-processing fashion, to build systems that have fascinating physical properties as well as mechanical and logical response. This work was led by Dr Lorenzo Di Michele (now Imperial College London) and a review of a large body of papers is in press, preprint here .
Cell Membranes
|
|
An erythrocyte (red blood cell) is "grabbed" at opposite ends by a pair of colloidal particles held in optical traps. The cell is dynamically stretched, measuring its mechanical properties. |
We have been studying the Red Blood Cell, which has a relatively simple membrane structure made of a phospholipid bilayer tethered to a thin cortical network of semiflexible filaments. We have observed very complex dynamics upon stretching the cell, which we have interpreted as a stress induced remodeling of the cytoskeletal network. This has also linked to the study of blood stage malaria host-pathogen interactions.
Biological applications of Optical Traps
|
|
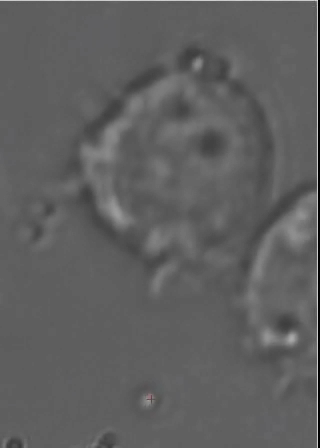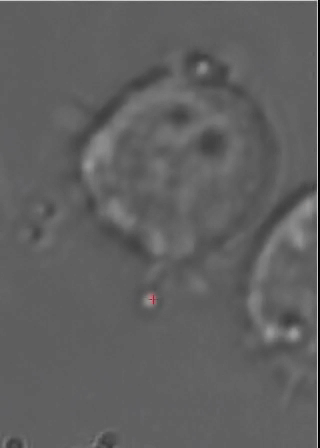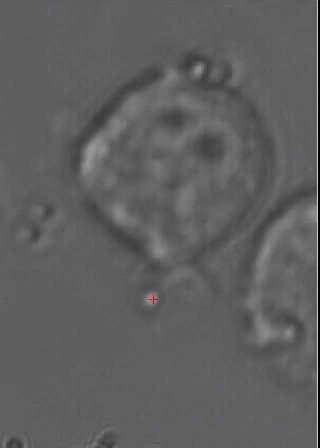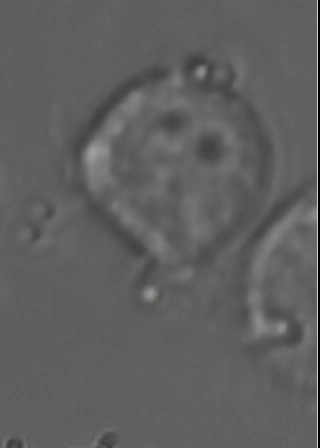
This animated gif shows a colloidal bead being dragged to the membrane of a macrophage cell. The interaction with beads of different coatings are being studied, as well as with bacteria. |
Optical Traps allow to move objects in solution (typically cells or colloidal particles), and to exert forces of the order of a few tens of pico-Newtons. We have a very advanced optical trap system which is easily programmed to perform sets of measurements, or to automatically perform certain "actions" based on video feedback from the experiment. This powerful instrument is being used also in collaboration with a few biologists, to study interesting problems such as cell infection by bacteria and other parasites.
4. Physical aspects of blood stage malaria
Malaria is one of the most common infectious diseases, affects 300 million people each year, causes nearly 1 million deaths, primarily children. Its elimination is hampered by the lack of an effective vaccine, and the recurrent evolution of parasites resistant to frontline drugs. In the blood stage of malaria, one parasite (P. falciparum merozoite) invades a red blood cell (RBC), then grows by repeated clonal divisions over about 48 hours, subverting the normal metabolism of the RBC: digesting haemoglobin, the merozoites release toxic heme groups. The parasites crystallise the heme into hemozoin. The metabolism of haemoglobin, and the formation of hemozoin crystals, are specific to this family of parasites and essential to their survival, hence these have been the targets for most malaria drugs up to now; current research is turning the focus also on invasion. From typically a single infection, a merozoite multiplies over rounds of replication, with up to 20 parasites egressing into the bloodstream each generation. Here, the merozoites have a very short time (about a minute) to adhere and invade a new host cell, and start a new round of clonal growth. This process, repeated over weeks, leads to high fractions of infected RBC and huge numbers of parasites. This is at the root of most human symptoms (e.g. anaemia and haemorrhage) and mortality. Building on three recent Physics PhD studies with me, a research project (EPSRC, funded 2018-2019) is now allowing us to develop low-cost microscopes in house, with similar optical specification to high end commercial units. This is essential to increase throughput and enable us The blood stage of malaria can be replicated in the laboratory as a cell culture. In the last decade we have pushed the frontier of direct imaging and micromanipulation to provide single-cell information, which can be crucial, beyond from bulk methods, to disentangle the origin of population behaviours. Live single cell imaging is providing a new class of information which we and others refer to as "invasion phenotypes". Our most recent work has been with J.Rayner (Wellcome Trust Sanger Institute) and other malaria biologists (T.Tiffert, V.Lew), and focused on the parasite adhesion and invasion stages. We have found strong evidence of the role of RBC tension in regulating the probability of a successful parasite invasion.
5. General Experimental Methodologies
A theme that spans across all our activities is the extraction of maximum information from image and video data. Microrheology is an example of this: the motion of tracer particles is tracked, and this information characterises the material in which the particles are embedded. Applications of this in our group are to study the mechanics of the cell cytoskeleton and the process of gelation in polymer and particulate systems. We are also actively developing new image analysis, for example for detection of cell edges and cell tracking.
A second general theme is instrument automation. We aim to automate as much as possible all our experiments, in order
to improve data acquisition, and in some cases to enable experiments that it would simply not be possible to run manually. The optical tweezers and our imaging microscopes can be run through a custom scripting language, which includes the possibility of feedback control via real time video analysis.
A third general theme is microfluidics. We are very happy to make use of the University's Nanoscience Center, as well as facilities in our Physics Department, and to collaborate with Kevin Dorfman at the University of Minnesota. We can make micron scale incubators for continuous and controlled growth of bacteria, and we are developing devices to study other unicellular organisms.


