|
|
||||

|

|

|
Professor Athene Donald: ResearchMy early research focussed on synthetic polymers, but in later years rather little of my current work was on this class of materials with an increasing move towards biological problems. Substantial efforts addressed starch and food physics; the development of Environmental Scanning Electron Microscopy; protein aggregation and cellular biophysics. I am no longer taking on new students or postdocs.Protein aggregation
Protein aggregation is relevant to both the texture of food (eg using the protein beta lactoglobulin) and disease (eg A beta aggregation). We seek to find generic behaviour, so that the factors that drive aggregation - particularly at the suprafibrillar level where spherulites are found to form (see image) - can be teased out. We are exploring the effect of electrostatic interactions and shear (as mediated by salt and pH) on the aggregates that form. Under a recent collaboration with Dr J Sharp and Professor C Roberts in Nottingham we mapped out the different conditions under which different types of aggregates formed. We are now exploring the role of flow, using microfluidics in collaboration with Drs Ulrich Keyser and Stefano Pagliari from BSS. Additionally, in a collaboratin with Professor Paul van der Shoot (Eindhoven) and Dr Hans Tromp (Nizo) in The Netherlands, we are exploring what happens in highly concentrated protein solutions. MRH Krebs, EHC Bromley and AM Donald - 2005. J Struct Biol, 149, 30-37. The binding of thioflavin T to amyloid fibrils: Localisation and implications. MRH Krebs, ECH Bromley and AM Donald 2005. Biophys J 88 2013-21. The mechanism of spherulite formation by bovine insulin amyloid fibrils. EHC Bromley, MRH Krebs and AM Donald 2006. EPJE 21 145-52. Mechanisms of structure formation in beta lactoglobulin near the isoelectric point. MRH Krebs, KR Domike and AM Donald - 2009. Biochem Trans 37, 682-6. Protein aggregation: more than just fibrils. C Exley, E House, JF Collingwood, MR Davidson, D Cannon and AM Donald - 2010. J Alzheimers Disease 20 1159-65. Spherulites in Abeta 42 in vitro and in Alzheimers Disease. V Fodera and AM Donald - 2010. EPJE 33 273-82. Tracking the heterogeneous distribution of amyloid spherulites and their population balance with free fibrils. MI Smith, V Fodera, JS Sharp, CJ Roberts and AM Donald 2012. Coll and Surf B. 89, 216-222 Factors affecting the formation of insulin amyloid spherulites. V Fodera, S Pagliari, O Otto, UF Keyser and AM Donald 2012. J Phys Chem Lett 3 2083-7. Microfluidics reveal a flow-induced large scale polymorphism of protein aggregates. FC Christie, AM Donald and OA Scherman - 2013. Biopolymers 100 Special Issue 289-289. Inhibition of human insulin aggregation through supramolecular host-guest interactions. V Fodera, A Zaccone, M Lattauda and AM Donald - 2013. PRL 111 108105. Electrostatics controls the formation of amyloid superstructures in protein aggregation. V Foderΰ, V Vetri , TS Wind, W Noppe , C Cornett, AM Donald A.M., L Morozova-Roche and B Vestergaard - 2014. J Phys Chem Lett 5, 32543258. Observation of the Early Structural Changes Leading to the Formation of Protein Superstructures. J Ioannou, Donald AM and Tromp H 2015. Food Hydrocolloids 46 216-225. Characterising the secondary structure changes occurring in high density systems of BLG dissolved in aqueous pH3 buffer. Cellular biophysics
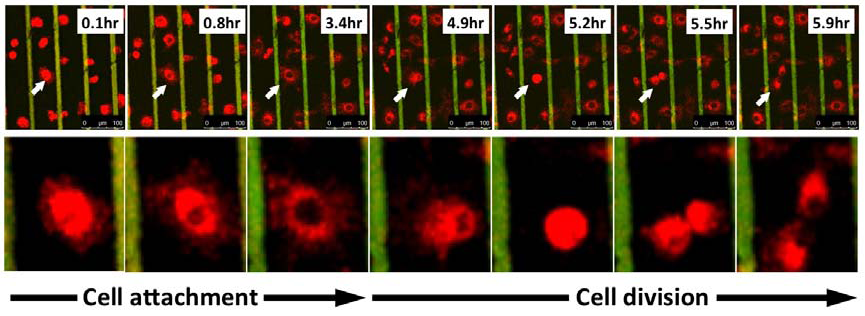
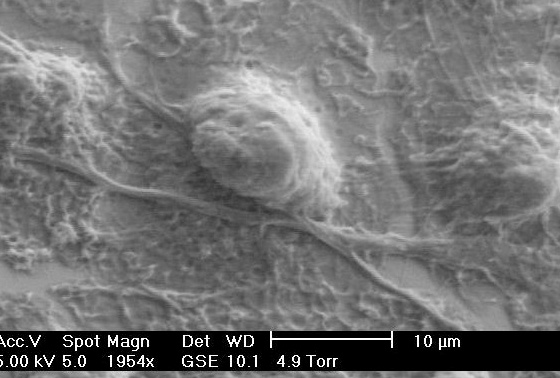
The majority of our work on cellular biophysics has concentrated on how 3T3 cells behave on ridge-groove patterned substrates. We have examined their spreading across patterns of different sizes;we have looked at the anisotropy of their motion on the ridges (collaboration with Dr G Battaglia, Sheffield); and we are looking at how the pattern influences mitosis(collaboration with Dr V Draviam, Genetics). We are also using microrheology (see below) to characterise the viscoelastic response inside the cell. PM Stevenson and AM Donald, - 2009. Langmuir 25 367-76. Identification of three regimes of behaviour for cell attachment on topographically patterned substrates. C Picard and AM Donald 2009. EPJE 30, 127-34. The impact of environmental changes on the microrheology of adherent cells. C Picard, V Hearnden, M Massignani, S Achouri, G Battaglia, S MacNeil, and A Donald 2010. BioTechniques 48, 135-138 A micro-incubator for cell and tissue imaging. KS Kung, I Canton, M Massignani, G Battaglia, AM Donald 2011.EPJE 34, 23-32. The development of anisotropic behaviours of 3T3 fibroblasts on microgrooved patterns. AM Corrigan, RL Shrestha, I Zulkipli, N Hiroi,, Y Liu, N Tamura, B Yang, Bing, J Patel, A Funahashi, A Donald and VM Draviam, - 2013. Cell Cycle 12 2643-2655. Automated tracking of mitotic spindle pole positions shows that LGN is required for spindle rotation but not orientation maintenance. C-K Huang and AM Donald 2015. Interface 12 20141064. Revealing the dependence of cell spreading kinetics on its spreading morphology using microcontact printed fibronectin patterns. Environmental scanning electron microscopy (ESEM)
The Environmental Scanning Electron Microscope (ESEM), sometimes referred to as low vacuum SEM, is the latest version of one of the most powerful analytic tools available to scientists.The low vacuum environment in the sample chamber (up to 1500 Pa) permits examination of samples in their natural state (wet, hydrated, uncoated, etc) without the need for conventional preparation techniques. We have been developing the technique for the last 15 years, currently examining conditions to image a range of biological samples. We also use in situ focussed ion beam milling to cut sections both into a bulk sample and also to make ultra-thin sections suitable for TEM eg through photovoltaic devices. KA Dragnevski and AM Donald - 2008. Coll and Surf A 317 551-6. Film formation mechanism of novel acrylic latex for solvent-free architectural coatings. S Kirk, JN Skepper and AM Donald 2009. J Micros 233, 205-24. Application of VPSEM to determine biological surface structure. J Benawara and AM Donald 2009. J Micros 234 89-94. Developing dual beam methodologies for the study of heterogeneous polymer-based systems. T Zheng, K.W. Waldron, A. M. Donald 2009. Planta 230 1105-13. Investigation of viability of onion epidermal tissue in the environmental scanning electron microscopy . D Waller, DJ Stokes and AM Donald- 2010. EPJ App Phys 51, 10901. Investigating sublimation and the effect of an imaging gas in a VPSEM J E McGregor and AM Donald 2010. J Micros 239 135-141. ESEM imaging of dynamic biological processes: the closure of stomatal pores. N Thomson, K Channon, N A Mokhtar, L Staniewicz, R Rai, I Roy, A MDonald, D Summer and E Sivaniah - 2011. Scanning 33 59-68. Imaging internal features of whole, unfixed bacteria. L Staniewicz, AM Donald, DJ Stokes, N Thomson, E Sivaniah, A Grant , D Bulmer and CM Anjam Khan 2012 Scanning 34 237-46. The application of STEM and in-situ controlled dehydration to bacterial systems using ESEM. JE McGregor, LTL Staniewicz, SE Guthrie (Nee Kirk) and AM Donald- 2013. Methods in molecular biology (Clifton, N.J.) 931 493-516. Environmental scanning electron microscopy in cell biology. Microrheology
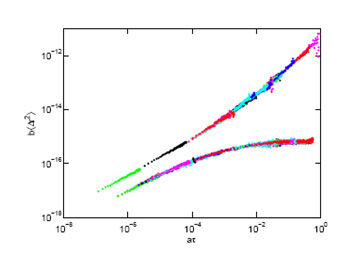
We use (passive) particle tracking microrheology to characterise soft matter samples ranging from clays to protein networks. We are particularly interested in following gelation in real time: microrheology provides an excellent way to measure the gel time accurately without invasively changing the network structure and have studied this both in peptide and protein gels. We are also interested in developing these approaches for intracellular studies (see above). IA Hasnain and AM Donald 2006. PRE 73 031901. Microrheological characterisation of anisotropic materials. HA Houghton, IA Hasnain and AM Donald, - 2008. EPJE 25 119-127. Particle tracking to reveal gelation of hectorite dispersions. AM Corrigan and AM Donald, 2009. EPJE 28 457-52 . Particle tracking microrheology of gel-forming amyloid fibril networks. C Picard and AM Donald 2009. EPJE 30, 127-34. The impact of environmental changes on the microrheology of adherent cells. AM Corrigan and AM Donald 2010. Soft Matter 6 4105-4111. Lengthscale Dependence of Apparent Critical Exponents Determined by Vibration-corrected Two-particle Microrheology. A Aufderhorst- Roberts, W Frith and AM Donald 2012. Soft Matter 8 5940-6. Micro-Scale Kinetics and Heterogeneity of a pH Triggered Hydrogel A Aufderhorst-Roberts, WJ Frith, M Kirkland and AM Donald - 2014. Langmuir 30 4483-92. Microrheology and microstructure of Fmoc-derivative hydrogels. A Aufderhorst-Roberts, WJ Frith and AM Donald - 2014. EPJE 37 article 44. A microrheological study of hydrogel kinetics and micro-heterogeneity. WJ Frith, AM Donald, DJ Adams, A Aufderhorst- Roberts 2015. J Non-Newtonian Fluid Mech 222 104-11. Gels formed from amino-acid derivatives, their novel rheology as probed by bulk and particle tracking rheological methods. Polymer morphology
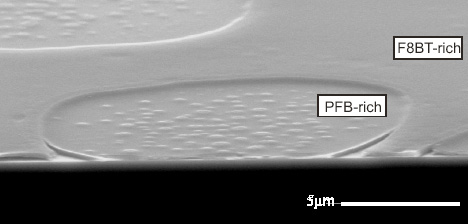
We have studied polymer morphology via ESEM and other microscopies. The main current project is part of the UKOPV consortium , continuing as a collaboration between Sheffield University and ourselves,which focuses on model organic photovoltaic (OPV) polymer blend systems between conjugated polymers and PCBM, with a view to gaining a comprehensive understanding of the intrinsic physical and chemical mechanisms that determine the ultimate device performance. The current project considers films produced by electrospraying and their long-term durability. Our part of the project uses predominantly electron microscopy (including focussed ion beam milling to produce cross-sections through devices) for characterisation, as well as forming part of the team at Diamond for synchrotron studies. T Wang, ADF Dunbar, PA Staniec, AJ Pearson, PE Hopkinson, S Lilliu, JE MacDonald, C Pizzey, NJ Rerrill, AM Donald, AJ Ryan, RAL Jones and DG Lidzey 2010. Soft Matter 6 4128-4134.The development of nanoscale morphology in polymer:fullerene photovoltaic blends during solvent casting. PA Staniec, AJ Parnell, ADF Dunbar, H Yi, AJ Pearson, T Wang, PE Hopkinson, C Kinane, RM Dalgleish, AM Donald, AJ Ryan, RAL Jones and DG Lidzey -2011. Adv Energy Mat 1 499-504. The nanoscale composition of a PCDTBT: PCBM photovoltaic blend. PE Hopkinson, PA Staniec, AJ Pearson, ADF Dunbar, T Wang, AJ Ryan, RAL Jones, DG Lidzey and AM Donald 2011. Macromols 44 2908-17. A phase diagram of the P3HT:PCBM organic photovoltaic system: Implications for device processing and performance. AJ Parnell, AJ Cadby, OO Mykhaylyk, ADF Dunbar, PE Hopkinson, AM Donald and RAL Jones. Macromolecules 44, 6503-6508. The Nanoscale Phase Separation of P3HT PCBM Thick Films as measured by Small Angle X-ray Scattering. AJ Pearson, PE Hopkinson, T Wang, PA Staniec, RAL Jones, AM Donald and DG Lidzey 2012. Macromols 44, 1499-1508. Rationalising the thermal annealing temperature for P3HT:PCBM blends. T Wang, NW Scarratt, H Yi, ADF. Dunbar, AJ Pearson, DC Watters, TS Glen, AC Brook, J Kingsley, AR Buckley, MWA Skoda, AM Donald, RAL Jones, AIraqi and DG Lidzey -2013. Adv Energy Mat 3 505-12. Fabricating high performance, donor-acceptor copolymer solar cells by spray-coating in air. TS Glen, NW Scarratt, H Yi, A Iraqi, T Wang, J Kinglsey, AR Buckley, DG Lidzey and AM Donald -2015. Solar Energy Mat Solar Cells 140 25-32. Grain size dependence of degradation of aluminium/calcium cathodesi n organic solar cells following exposure to humid air. RC Masters, AJ Pearson, TS Glen, FC Sasam, LT Li, M Dapor, AM Donald, DG Lidzey and C Rodenbury 2015. Nature Comms 6 6928. Sub-nanometer resolution imaging of polymer-fullerene photovoltaic blends using energy-filtered scanning electron microscopy. | |||||||||||||||