Biophotonics and the Physics of Pluripotency | ||
|
Dr. Kevin Chalut Royal Society Research Fellow |

|
|
Please contact me about an interdisciplinary postdoc opportunity (October 2012).
In the last 50 years, the chemical world of the biological cell has been largely revealed. But cells exist within an intrinsically physical environment - think of blood cells squeezing through vessels - and are inherently physical objects - consider the cytoskeleton, which gives a cell its shape, as an out-of-equilibrium polymer network. We are using novel biotechnology, including laser trapping and digital holographic microscopy, to study the physics of cells in order to better understand how they work. We are primarilly investigating pluripotent stem cells, which have a superfically simple, belying a misunderstood and highly important, function. Through cell division, distributions of pluripotent stem cells self-renew while simultaneously transforming into the eventual tissue cells necessary for an organism. Somehow, using a single genome, you have progressed from a zygote to what you are. We are showing that physical processes - such as the dynamics of chromatin, i.e. the physical structure of the genome - play a significant role in this development.
BiotechnologyWe are pursuing biotechnology that can assess physical phenotypes, such as subcellular structure - how cellular material is organised - and cell/nuclear mechanics - how soft the cell and nucleus are. We are most interested in marker-free techniques that require no external labelling of biological samples. These techniques include digital holographic microscopy, which is a quantitative phase microscopy technique capable of sensitive discrimination of small subcellular structures. We are currently implementing tomographic techniques in order to assess subcellular structure in 3D with high resolution. Our biotechnology techniques also include tools such as optical stretching and nanoparticle tracking. 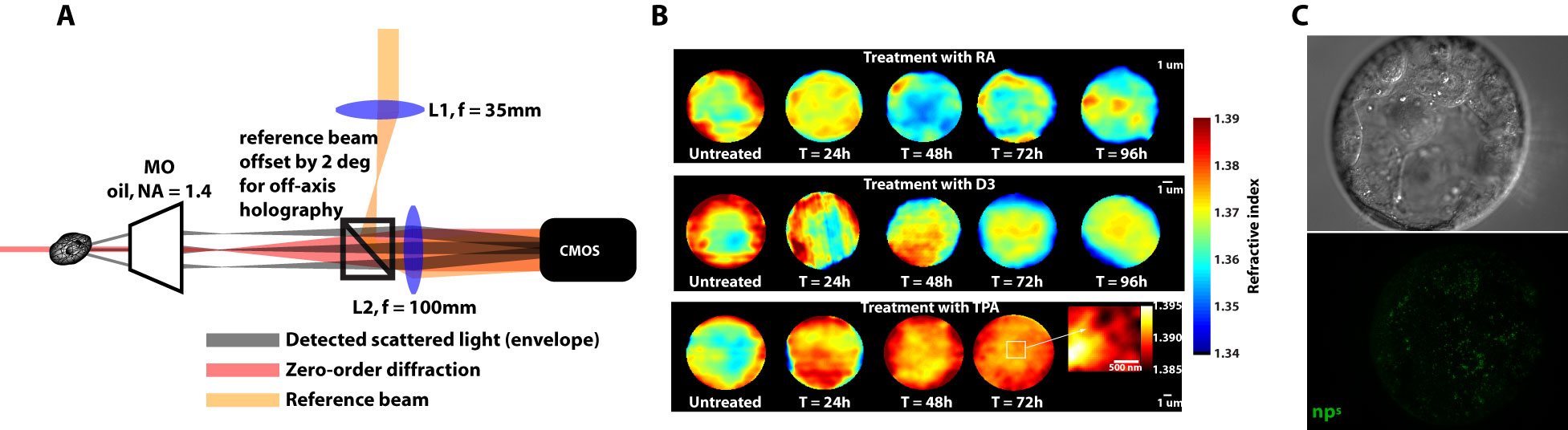
(A) The DHM is a novel imaging technique we use to deduce refractive index, which gives us marker-free subcellular structure, as see in (B), which are refractive index distributions of three different differentiation routines in myeloid precursor cells. (C) Nanoparticles are injected into embryos in order to deduce nuclear mechanics and micro-organisation. |
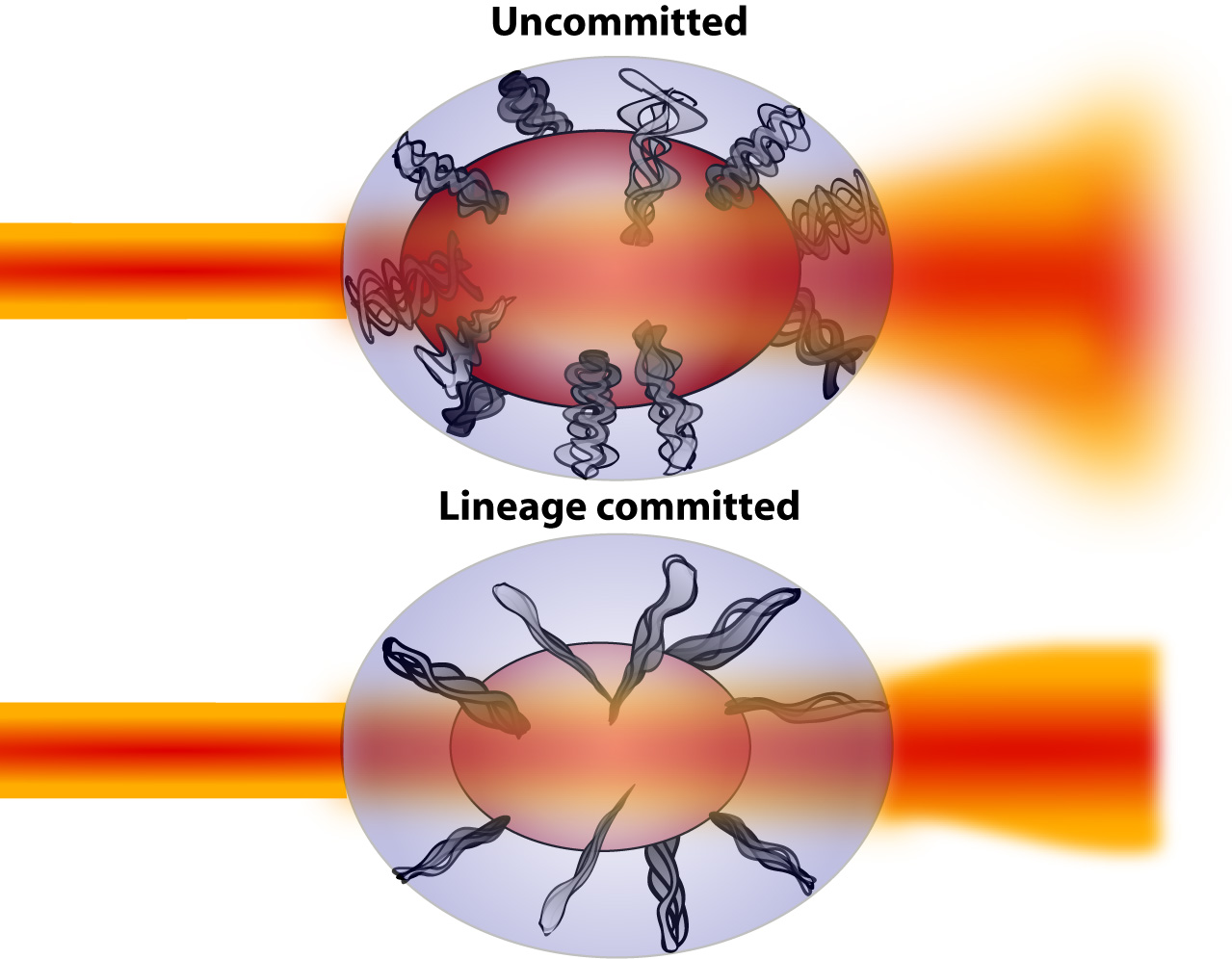
During the process of differentiation, during which a cell commits to a particular lineage, physical alterations occur. These physical alterations cause changes in the way the cell interacts with light. We hope to quantify and characterise these changes to better understand cells and how to monitor their behaviour. |
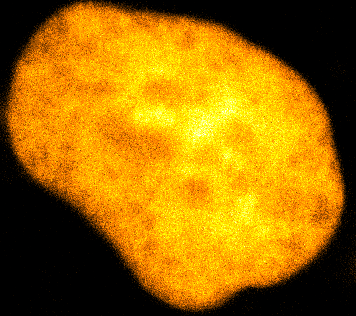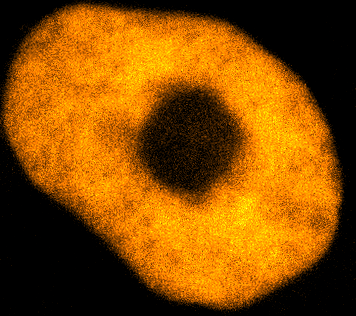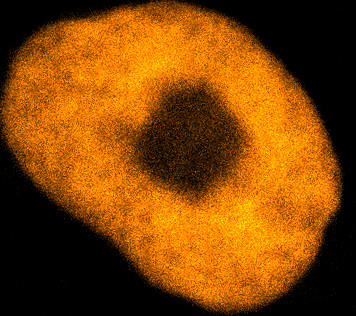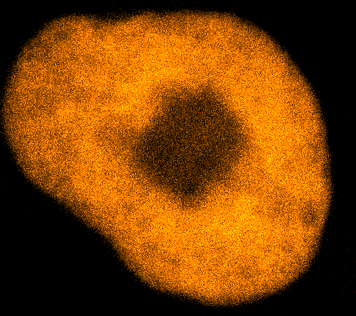
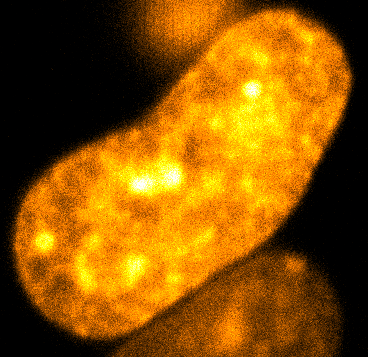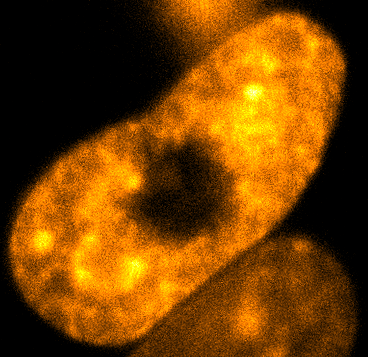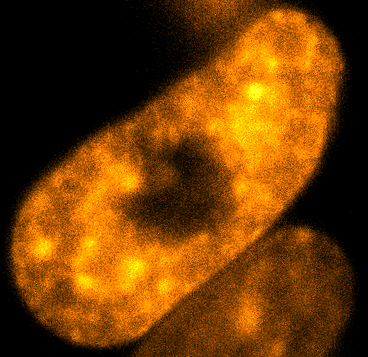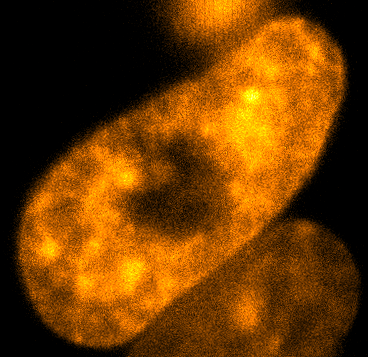
Physical phenotypingWe will use our developed biotechnology to establish physical phenotypes of cell function, particularly pluripotency and differentiation. The physical phenotypes we will mainly explore are nuclear mechanics and micro-organisation. We are particularly interested in the role the remodelling of chromatin - the machinery in the nucleus of which chromosomes are built, comprising histone proteins and DNA which wraps around them - plays in the function of pluripotent stem cells. Chromatin is highly dynamic: in order to control accessibility to the genome, it condenses or decondenses, and moves throughout the nucleus as a a cell fulfils its function. The physical phenotypes we will study focus on more big-picture views of cell behaviour to generate a broader understanding of changes in cell function and will impact both cell biology and medicine. One example of this is the process seen below, where an ES cell nucleus is transfected with histone H2B-RFP, and then bleached in the middle. By watching the movement of unbleached proteins, we can deduce the kinetics of chromatin within then nucleus.
|
|
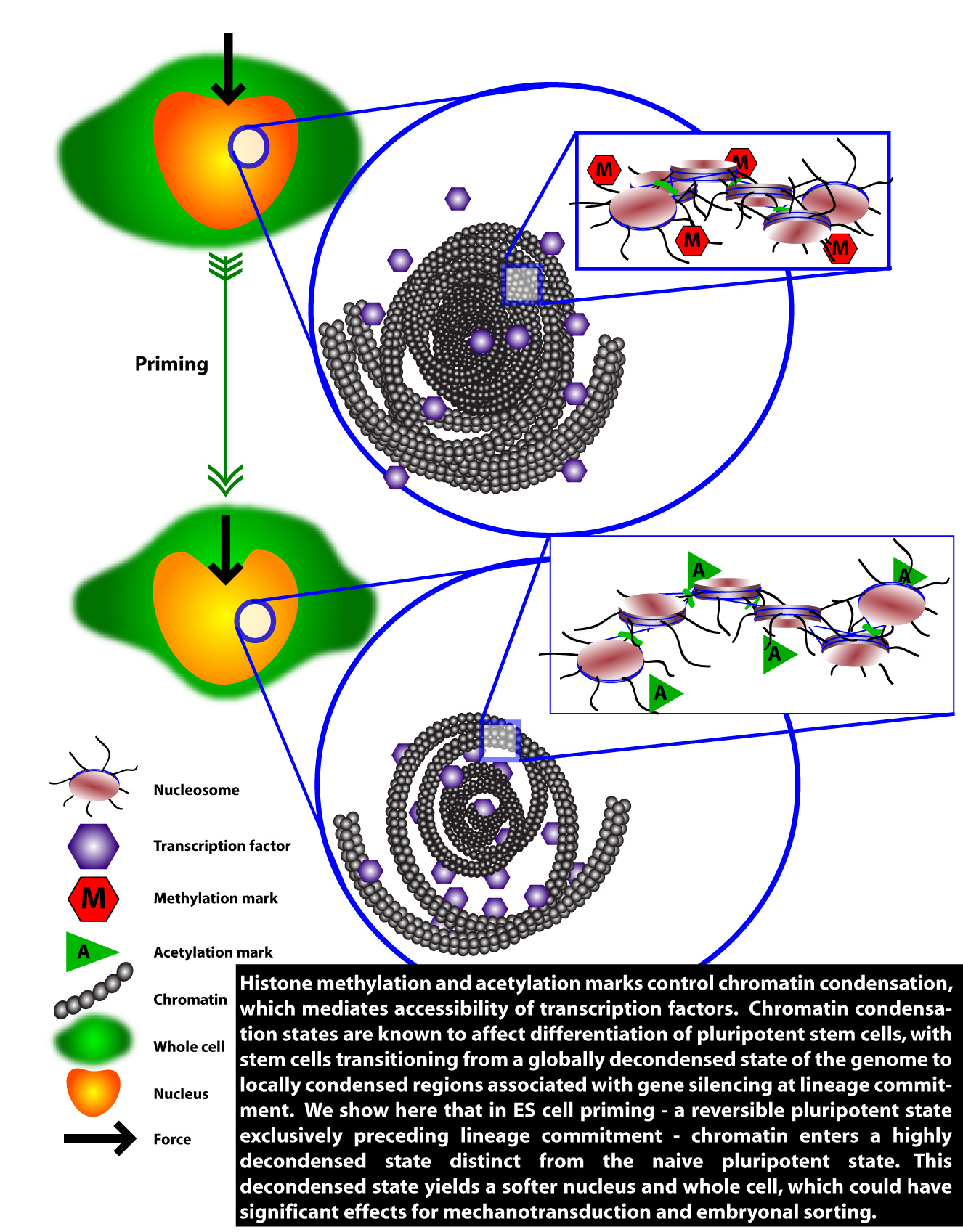
System level pluripotencyPluripotent stem cells mediate development through a combination of factors, including regulating access to the genome. This regulation occurs largely through epigenetic processes, which are chemical marks, covalently bonded, inserted into genes to regulate gene expression. These epigenetic processes have a physical consequence. Certain epigenetic marks, such as methylases, tend to condense chromatin, while other epigenetic marks, such as acetylases, tend to decondense chromatin. This opening and closing of chromatin regulates access to the genome by transcription factors. We seek a systematic understanding of how epigenetic processes reverberate through the cell at a system level, starting from structural changes to chromatin. We have shown that pluripotency is affected by global condensation and decondensation of chromatin, and that this causes changes in cell and nuclear mechanics. The important question is: does this affect cell fate, and do these processes drive embryonic development? We think they do, almost certainly in cell sorting in the embryo (regulated by cell and nuclear mechanics), and also probably at the transcriptional level by mechanotransduction (the process by which forces are integrated by the cell and turned into changes in gene expression). We will be exploring pluripotency at a system level using our biotechnology toolbox and pysical phenotyping techniques. |