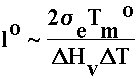· Polymer-polymer interaction energetically favourable. Then
<r2>1/2= N na with n<1/2.
The chain shrinks compared with the ideal case.
This applies for poor solvents, where polymer-polymer interactions preferred to polymer-solvent.
Both these situations apply when plenty of solvent molecules around ie dilute solutions.
· Do ideal <r2> 1/2= N 1/2a statistics every apply?
ie when no advantage or disadvantage to segments of the same chain coming into contact with each other? Yes!
a) In a 'q solvent' the solvent-polymer and polymer-polymer interactions are energetically the same. The chain obeys Gaussian statistics.
b) In a melt. An individual segment may interact with segments from the same or different one, but energetically the same, so Gaussian statistics obeyed again.
Distribution of Chain Lengths
In general there will be a distribution of chain lengths present (due to most synthesis methods).
The molecular weight distribution can be characterised in various ways.
Suppose there are ni molecules with DP Ni molecular weight Mi (where molecular weight is the number of monomers x the weight of one monomer).

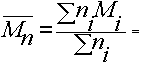 number average
molecular weight
number average
molecular weight

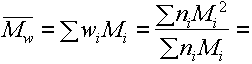 wt average molecular
weight
wt average molecular
weight
i.e these are different moments of the molecular weight distribution.
For a monodispers system Mw/Mn=1, but very hard to achieve. Best in practice ~ 1.03, and this can only be achieved for certain polymers and polymerisation routes.
In practice Mw/Mn~2 for many polymerisation routes, but for PE typically much broader.
Polymer Crystallisation
See eg DC Bassett Principles of Polymer Morphology, CUP.
Not all polymers can crystallise.
· Only polymers which are chemically and sterically regular can crystallise
ie isotactic and syndiotactic can, atactic cannot.
For copolymers (polymers containing more than one unit):
AABABBABABBAB – random copolymer – cannot.
ABABABABABABA - alternating - can.
(A)n(B)m - block - can.
· If a polymer molecule is branched, this impedes crystallinity.
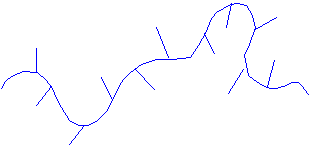 PE often has short chain branches.
PE often has short chain branches.
The length and frequency of these affect the ability to crystallise.
· Molecular weight also important.
Crystallisation temperature depends on chainlength. For isothermal crystallisations, only chains of a certain length can crystallise – get fractionation by molecular weight.
· No polymer is ever fully crystalline when prepared from solution or melt.
· Only examples of 100% crystalline polymer have been produced from epitaxially deposited monomer then polymerised in-situ.
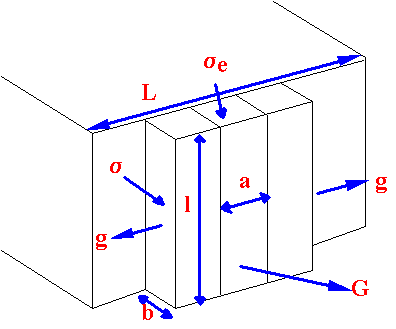
How do long, flexible chains crystallise?
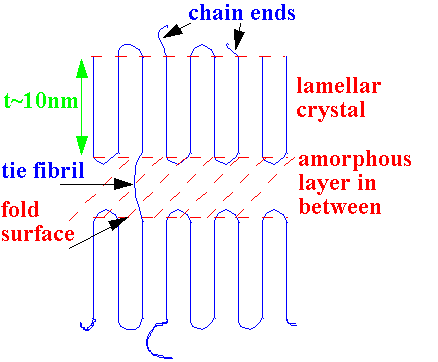
Chain
folded
crystal
What is the evidence for a folded chain structure?
Comes from transmission electron microscopy (TEM) images of single crystals which have been 'shadowed' by heavy metal atoms at an inclined angle.
Size of shadow can be related to thickness of crystal, ~10 nm – much less than total chain length.
However it is still not clearly understood why chains fold as they do.
Single crystals of type seen in TEM are atypical, since they can only be prepared from dilute solution.
However such crystals do permit electron diffraction to provide information on the crystal structure (usually orthorhombic for PE).
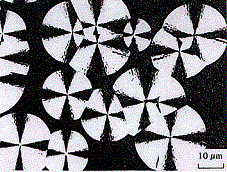
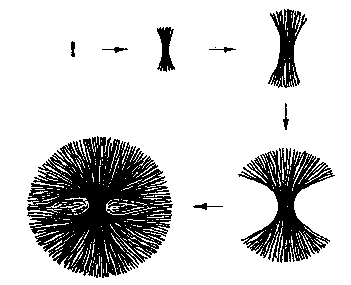
More typical structures are spherulites.