Crystalline Solids
Books
There are many good texts on crystalline solids which cover the defects we will be covering here, many of which will already be familiar to you from Solid State.
e.g
Kittel
Ashcroft and Mermin
Rosenberg
More specialist texts:
Hull and Bacon – Introduction to Dislocations
Ashby and Jones – Engineering Materials
Defects
Three basic (geometric) types:
· Point – vacancy, interstitial, substitutional
· Line – dislocations
· Plane – grain boundaries
Point Defects
1. Vacancy or Schottky Defect

Perfect Crystal Defect Crystal
Free energy Go Free energy G
More complicated in ionic crystals, where still need to maintain charge neutrality in the bulk.
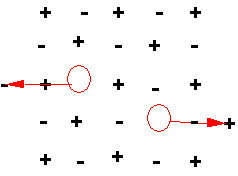 Positive and negative ions both move
to surface, leaving a pair of
vacancies.
Positive and negative ions both move
to surface, leaving a pair of
vacancies.
Defects will affect both optical and electronic properties.
In general, the energy of formation Ev depends on site to which atom moved.
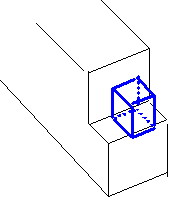
Ev lower if transferred to kink site
(crystal ledge) than perfect surface.
On average Ev corresponds to net breaking of ~1/2 neighbouring bonds
~1/2 latent heat of sublimation/atom
Ev~1eV
2) Interstitial vacancy – Frenkel defect
 |
Ionic crystal – 2 types
 |
More likely since cations tend to be smaller than anions Ţ lower associated strain energy.
Energy due to strain (non-ionic case)
Strain energy = 1/2 elastic constant x strain2 /vol
Define shear modulus G = 
Strain energy = 1/2 G g2 (or equivalently 1/2 tg)
If b = lattice parameter
Volume ~ b3
Strain ~1
ŢStrain energy = 1/2 Gb3
hence EFrenkel~ 5-6ev
Much larger than ESchottky and also EFrenkel> kBT
In general not thermodynamically stable, and won't be discussed further.
Equilibrium number of vacancies in monatomic crystal
(For complete discussion see Waldram, Theory of Thermodynamics)
Compute F for crystal with N atoms, n vacanices on N+n sites.
3 contributions to toal entropy
· Sc determined by density of states etc for given configuration of atoms.
· Sbµ number of bulk configurations
· Ssµ number of surface arrangements
And Sc= kBln gc(E)
Sb= kBln Wb
Ss= kBln Ws
At equilibrium
 = 0
= 0
where dFc = dE-TdSc-TdSs, the change in free energy when we move an atom from a particular bulk site to a particular surface site, without allowing lattice rearrangements to occur.
DFc ~ 6 nearest neighbour bond energies (since break on average 1/2 the bonds in the surface)
Now 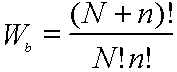
If 1 vacancy added Wb multiplied by
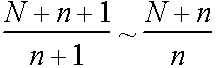
\ 
For large crystals dSs<<dSb
\ 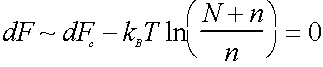
\n ~ N exp –dFc/kBT
This is generally quite small, but can become appreciable towards the melting point.
We will see later how vacancies are important for creep and diffusion
Dislocations – Line Defects
Dislocations were originally invoked to explain the discrepancy between theoretical shear stress and that experimentally determined, long before a dislocation was directly seen.
Theoretical Shear Stress
As two atom planes move past one another, the stress must increase and then decrease.

Assume a sinusoidal form for the variation of shear stress t with displacement x.
Shear stress 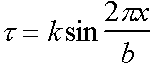 with k = const
with k = const
Near origin, slope is measure of elastic shear modulus G.
Hence, within this linear
regime  and
and
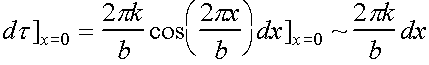
Ţ ![]() and therefore
and therefore 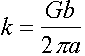
\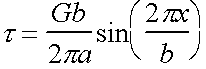
Maximum shear stress t0 is given by

Better models give to ~ G/30
Experiment shows this is far too high
e.g Copper G=4.6 GN m-2 Ţto = 0.72 GN m-2
Experimentally a
good single crystal gives to 100 kN m-2
Dislocations
The origin of the discrepancy between theory and experiment lies in the existence of dislocations.
Dislocations are characterised by their Burger's vectors. These represent the 'failure closure' in a Burger's circuit in imperfect (top) and perfect (bottom) crystal.
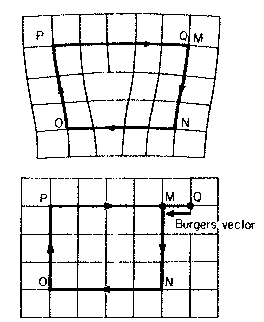
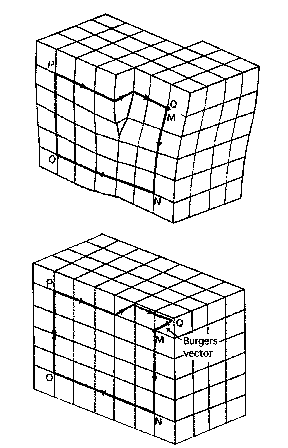
Edge Screw
Vectors describing dislocation line and Burger's vector are
Perpendicular Parallel
Dislocation Motion
Dislocations make a material softer because they permit crystals to deform without moving one entire crystal plane over the one below.
e.g. movement of edge dislocations
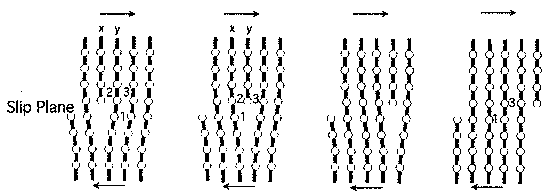
The slip (also known as glide) plane is the plane on which the dislocation moves.
The glide plane is defined by the vectors b and l.
This means edge dislocations have a unique glide plane, but screw dislocations do not and can move on a whole family of planes.